Thrombomodulin (TM) is a transmembrane protein that plays a pivotal role in maintaining microvascular health. Functions of TM include 1) binding thrombin and inhibiting its interaction with fibrinogen, 2) augmenting protein C function, and 3) down-regulating complement activation. Reduced TM function has been implicated in several thrombotic microangiopathies, including atypical hemolytic uremic syndrome and disseminated intravascular coagulation (DIC) (PMID 29866818). Previous in vitro studies have demonstrated that cell-free hemoglobin (Hb) mediates loss of TM from the endothelial surface which, in turn, is associated with a higher risk of kidney injury in patients with sickle cell disease (PMID 34929051). Infusion of intact, soluble TM may restore TM's function in the microvasculature, as observed in critically ill patients with DIC (PMID 17059423).
In transgenic sickle mice (Townes model, Jackson Laboratory; Bar Harbor, Maine), we investigated whether 1) cell-free Hb reduces TM function leading to glomerular endothelial and kidney injury and 2) if infusion of intact, soluble TM (Asahi Kasei Pharma ©; Tokyo, Japan) can restore TM function and reduce the toxic effects of cell-free Hb in the kidney. Sickle mice (4 male, 4 females per condition) were challenged with cell-free Hb (0.24g/kg intravenous [iv]) or normal saline followed 2 hours later by either infusion of TM (5mg/kg subcutaneous, 1mg/kg iv) or normal saline. Blood and urine samples were collected 24 hours after the challenge and the mice were sacrificed for histopathologic evaluation. In a separate set of similar experiments, sickle mice (3 males, 3 females per condition) received iv microbubbles (Vevo MicroMarker TM Contrast) 24 hours after the challenge to assess renal cortical blood flow by contrast-enhanced ultrasound (Vevo2100 system). Mean ± standard error of mean values are provided. Comparisons between Hb-only and Hb+TM conditions were made using the Mann-Whitney test.
Vasculopathy: Infusion of cell-free Hb resulted in decreased TM antithrombotic function within the glomerulus; infusion of TM 2 hours after the challenge restored these functions (fibrinogen relative fluorescent intensity [RFI]: control, 24.2 ± 0.9; Hb-only, 40.6 ± 1.7; Hb+TM: 26.8 ± 1.6) and anti-complement function (C3 RFI: control, 12.9 ± 0.4; Hb-only, 19.3 ± 1.4; Hb+TM, 14.1 ± 0.5) (P≤0.007). Circulating vascular injury biomarkers rose in the cell-free Hb challenged sickle mice and this was abated with TM rescue for p-selectin (control, 271 ± 25 ng/mL; Hb-only, 533 ± 47 ng/mL; Hb+TM, 356 ± 34 ng/mL), VEGF (control, 59 ± 4 pg/mL; Hb-only, 93 ± 7 pg/mL; Hb+TM 69 ± 3 pg/mL), and vWF (control: 91 ± 9 ng/mL, Hb-only: 299 ± 31 ng/mL, Hb+TM: 183 ± 22 ng/mL) (P≤0.01).
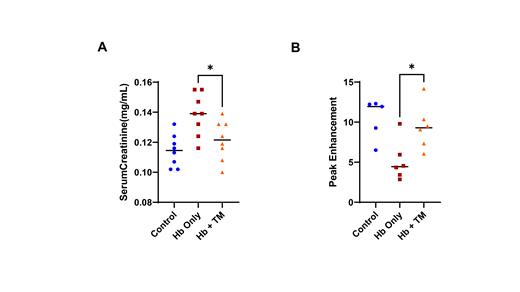
Kidney Damage: Urine biomarkers of tubular injury increased after the cell-free Hb challenge and improved with TM (KIM-1: control, 317 ± 66 pg/day; Hb-only, 1201 ± 142 pg/day; Hb+TM, 638 ± 78 pg/day; P=0.003) (NGAL: control, 234 ± 34 ng/day; Hb-only, 1624 ± 145 ng/day; Hb+TM, 767 ± 118 ng/day; P=0.003). An elevation in serum creatinine concentration is used to define acute kidney injury. The cell-free Hb challenge led to an acute rise in serum creatinine while the TM rescued mice had serum creatinine concentrations that were significantly lower than the Hb-only challenged mice (P=0.03) and similar to the control conditions (Figure 1A). Preliminary testing of TM administered 24 hours after the cell-free Hb challenge has also led to kidney protective effects as assessed by serum creatinine, p-selectin, and vWF (P < 0.05).
Cortical Blood Flow: Peak enhancement, a measure of relative blood volume, was reduced in the cell-free Hb challenged mice compared to control conditions by contrast-enhanced ultrasound. The TM-rescued mice had improved peak enhancement compared to the Hb-only treated mice (P=0.03) and similar to control conditions (Figure 1B).
In conclusion, cell-free Hb reduces TM function resulting in increased vascular and kidney injury and decreased cortical blood flow. Infusion of soluble TM 2 hours after the cell-free Hb challenge restores TM function, protects against vascular and kidney injury, and preserves cortical blood flow. Our data highlights TM as a potential candidate therapy to preserve vascular function and abate kidney injury in patients with sickle cell disease during severe vaso-occlusive or hyperhemolytic episodes, when concentrations of cell-free Hb increase several fold.
Disclosures
Gordeuk:GBT/Pfizer: Consultancy, Research Funding; CSL-Behring: Consultancy; Takeda: Consultancy; Emmaus: Consultancy, Research Funding; Incyte: Research Funding; Novartis: Research Funding; Modus Therapeutics: Consultancy; Forma: Consultancy, Research Funding. Saraf:GBT/Pfizer: Consultancy, Other: Advisory board, Research Funding, Speakers Bureau; Novartis: Consultancy, Other: Advisory board, Research Funding; Forma Therapeutics: Consultancy, Other: Advisory board, Research Funding; BEAM Therapeutics: Consultancy, Other: Advisory board; Agios: Consultancy, Other: Advisory board.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal